Biological Evaluation Plan and Report - MakroCareRegulatory, Clinical Consulting Services to Biopharma & Medical Device Companies | MakroCare

The Biological Evaluation Plan: An approach to the biological evaluation of medical devices with a focus on substance-based medical devices (November 2020) - Journal of Medical Device Regulation

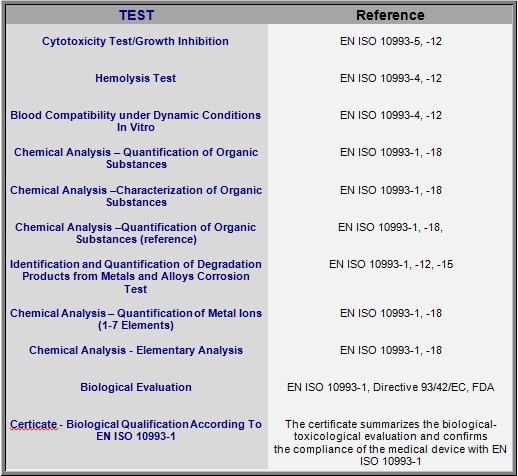
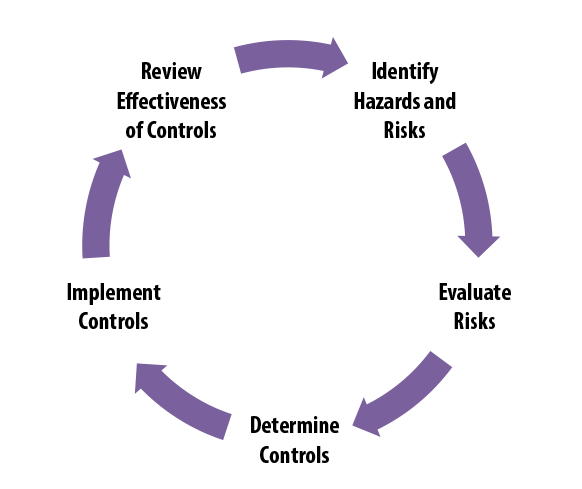
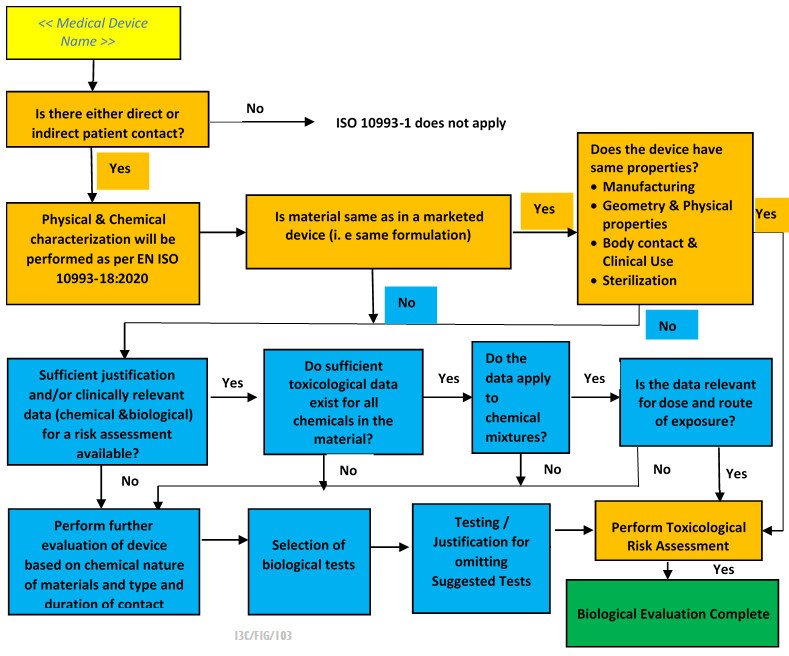
ISO 10993-1:2018(en), Biological evaluation of medical devices — Part 1: Evaluation and testing within a risk management process

Biological Evaluation Plan: A crucial first step in the Biocompatibility evaluation of a Med Device - YouTube

ISO 10993 Webinar Series: Importance of Biological Evaluation Plan & Common Biocompatibility Pitfalls (Part 7 of 7) | TÜV SÜD in India