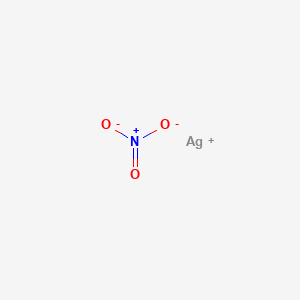
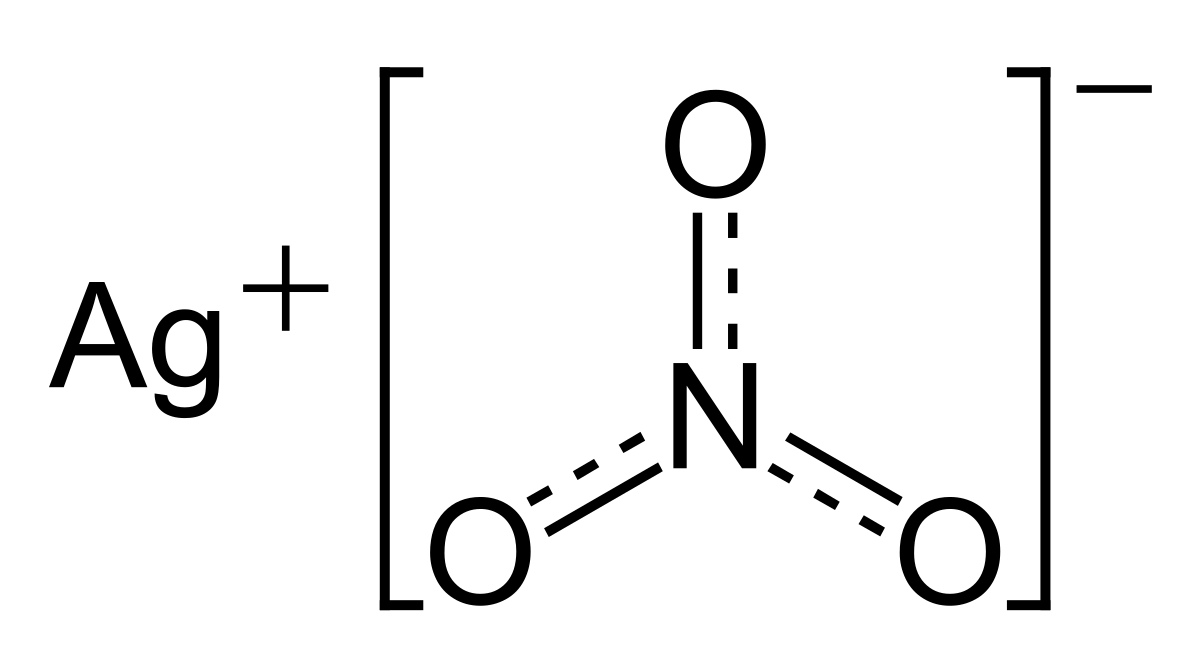
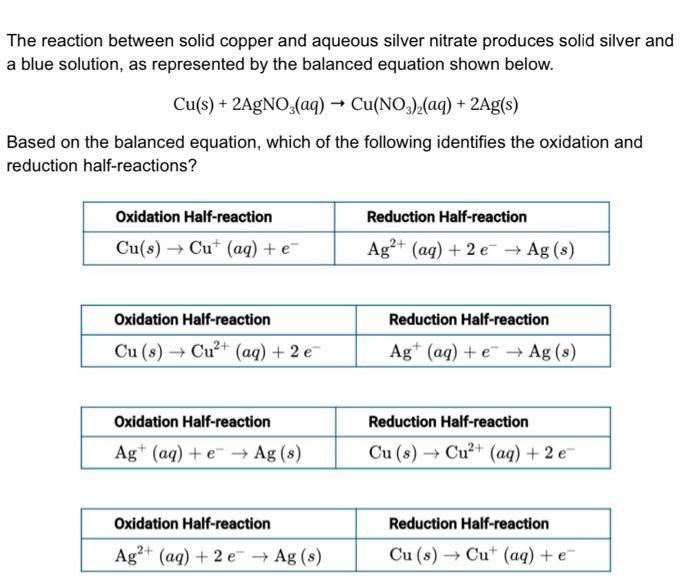
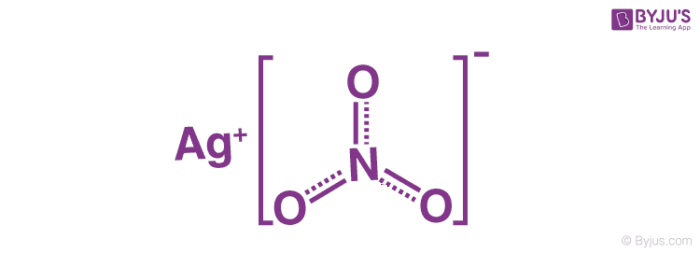
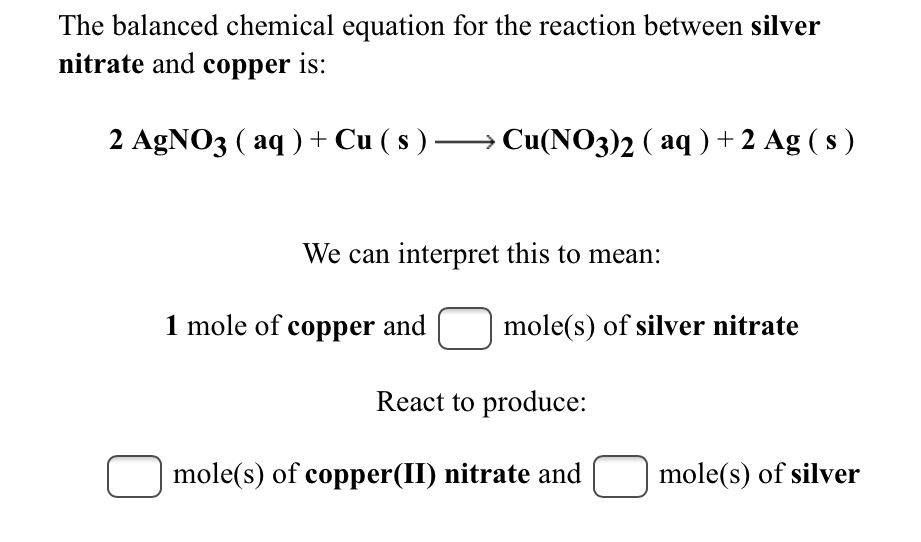
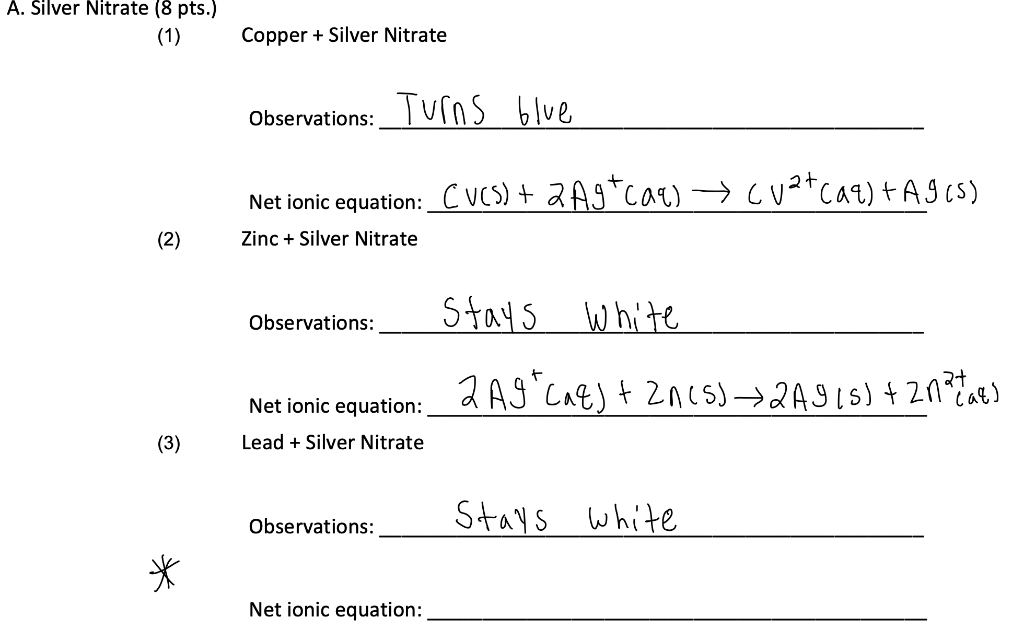
When copper is dipped in the solution of silver nitrate, the solution turns blue. Give the reason along with chemical equation?

Write the balanced chemical equations for the following reaction:Calcium hydroxide + Carbon dioxide → Calcium Carbonate + Water.
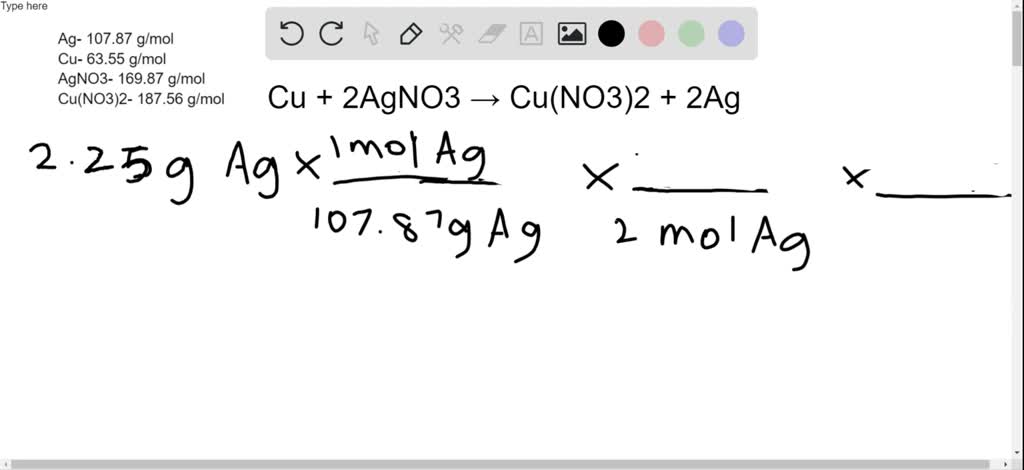
SOLVED:Copper reacts with silver nitrate through single replacement. a. If 2.25 g of silver are produced from the reaction, how many moles of copper(II) nitrate are also produced? b. How many moles