ISO 11137-2:2013, Third Edition: Sterilization of health care products - Radiation - Part 2: Establishing the sterilization dose: International Organization for Standardization: 9789267108568: Amazon.com: Books

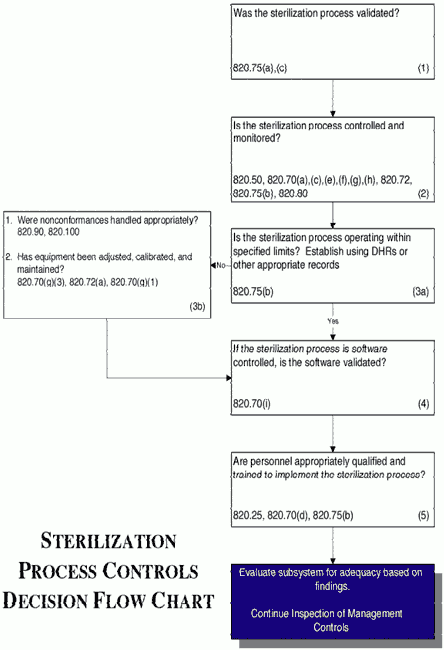
STERIS Applied Sterilization Technologies - Through locations in Europe, the United Kingdom and United States, our Radiation Technology Centers are dedicated to meeting the validation, dose audit, and research needs of our
Gamma-Irradiation: Sterilization Validation Approach for Allegro™ Single-Use Systems with Sterile Claim

Guide to Irradiation and Sterilization Validation of Single-Use Bioprocess Systems - BioProcess InternationalBioProcess International
Gamma-Irradiation: Sterilization Validation Approach for Allegro™ Single-Use Systems with Sterile Claim

Achieving sterility in biomedical and pharmaceutical products (part-II): radiation sterilization - ScienceDirect
Gamma Irradiation: Sterilization Validation Approach for Pall Biopharmaceutical Filters with Sterile Claim

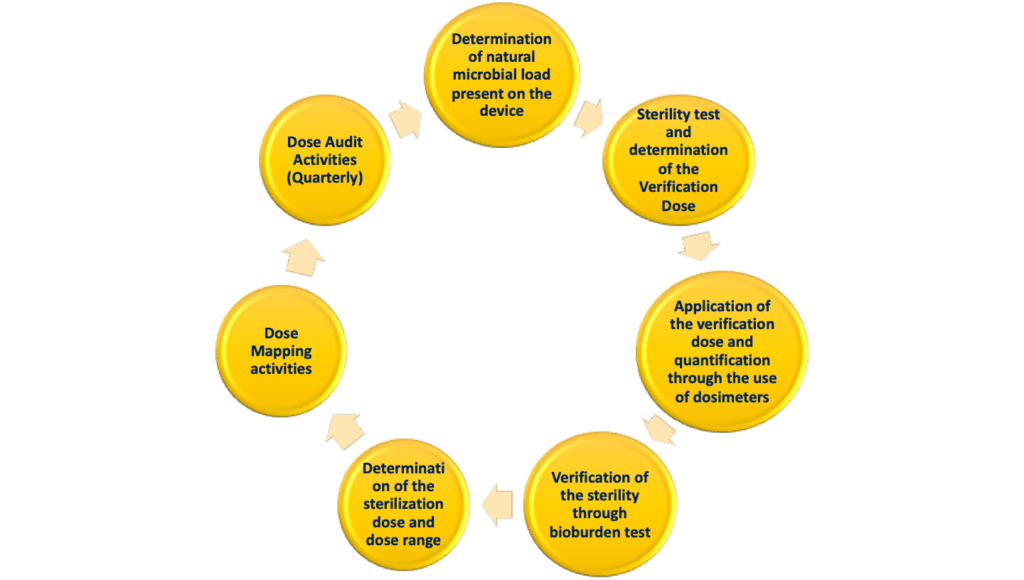
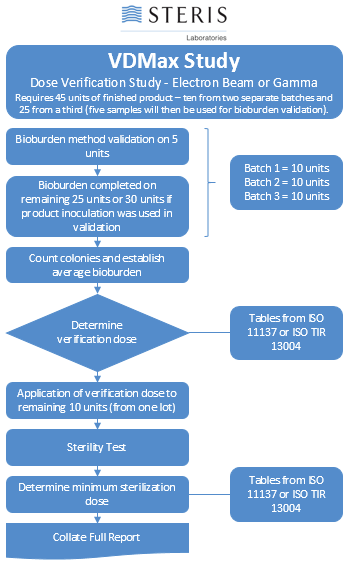
AAMI TIR35:2016 - Sterilization of health care products - Radiation sterilization - Product adoption and alternative sampling plans for verification dose experiments and sterilization dose audits.