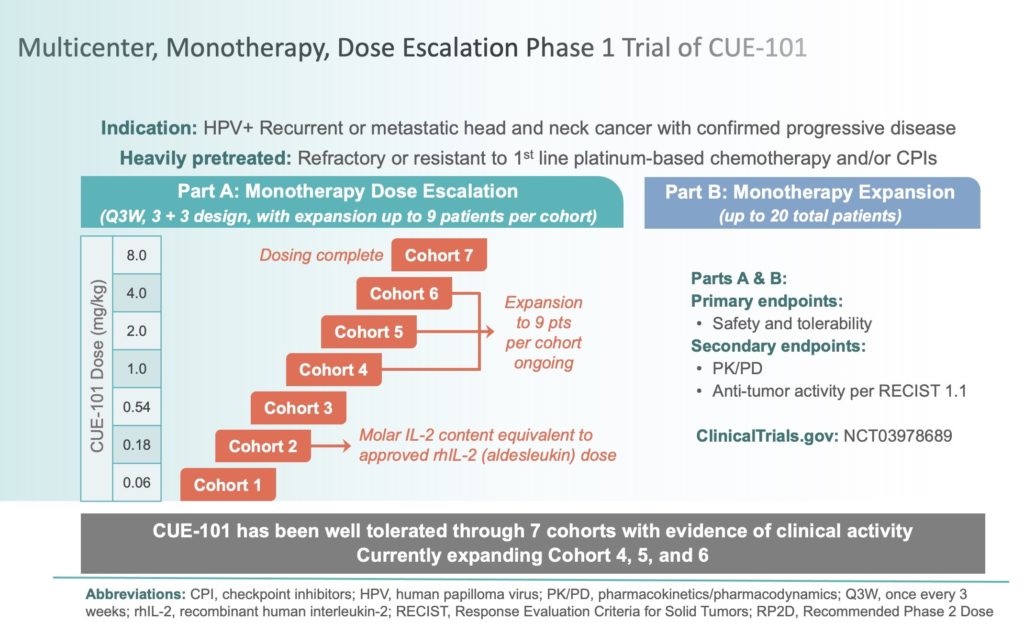
Dose escalation and expansion (phase Ia/Ib) study of GLS-010, a recombinant fully human antiprogrammed death-1 monoclonal antibody for advanced solid tumors or lymphoma - European Journal of Cancer

Moving Beyond 3+3: The Future of Clinical Trial Design | American Society of Clinical Oncology Educational Book

Innovative design for a phase 1 trial with intra-patient dose escalation: The Crotoxin study - ScienceDirect

Phase I dose-escalation study to determine the safety, tolerability, preliminary efficacy and pharmacokinetics of an intratumoral injection of tigilanol tiglate (EBC-46) - eBioMedicine

Dose intra‐subject escalation to an event (DIETE): A new method for phase 1 dose‐finding utilizing systematic intra‐subject dose escalation with application to T‐cell engagers - Xu - 2021 - Pharmaceutical Statistics -
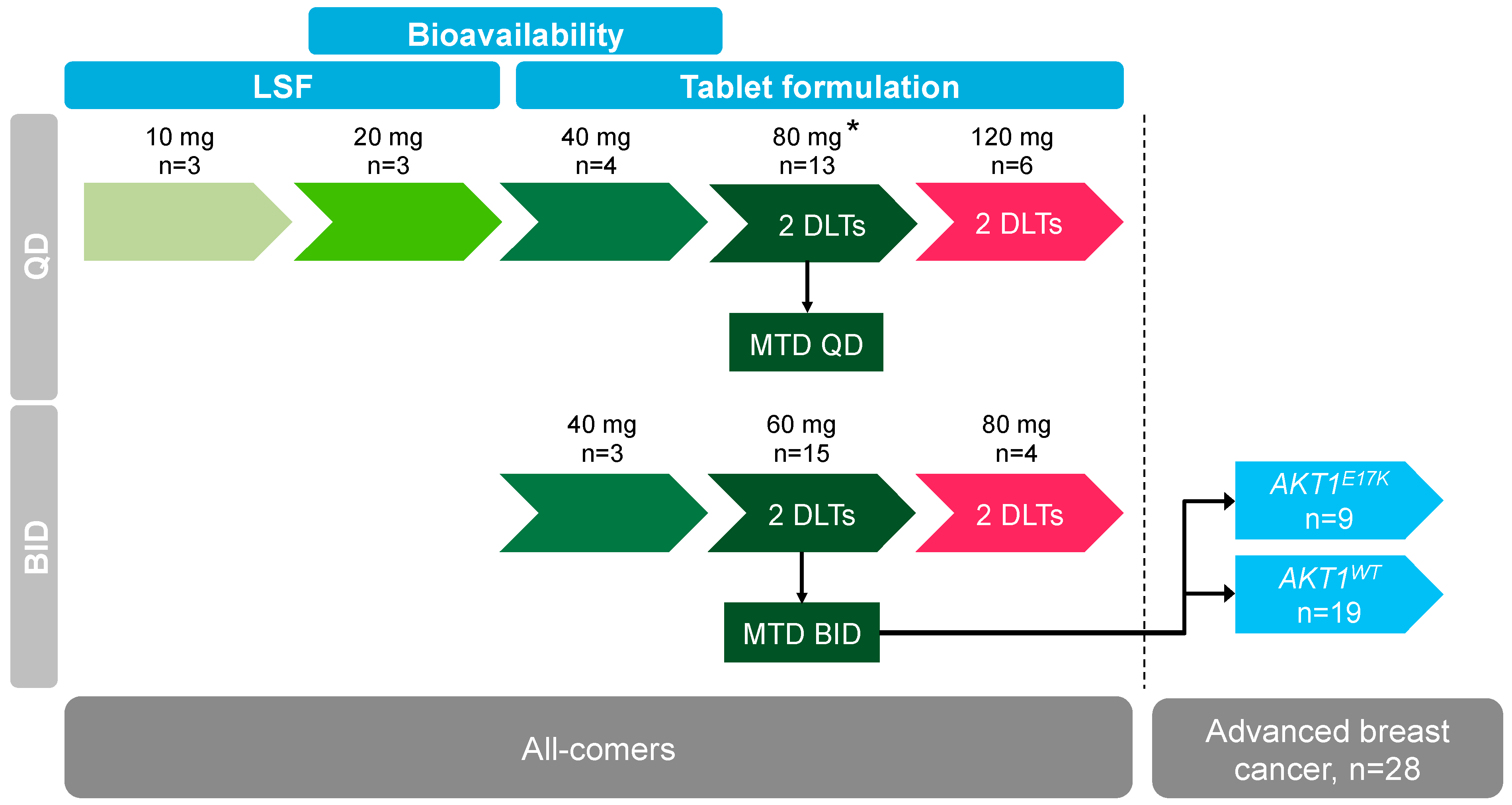
Cancers | Free Full-Text | Phase 1 Dose Escalation Study of the Allosteric AKT Inhibitor BAY 1125976 in Advanced Solid Cancer—Lack of Association between Activating AKT Mutation and AKT Inhibition-Derived Efficacy

Phase I/II Dose-Escalation Trial of Daratumumab for Relapsed/Refractory Multiple Myeloma | Research To Practice

Dose escalation. The dose escalation was performed in both intra and... | Download Scientific Diagram

Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial - The Lancet
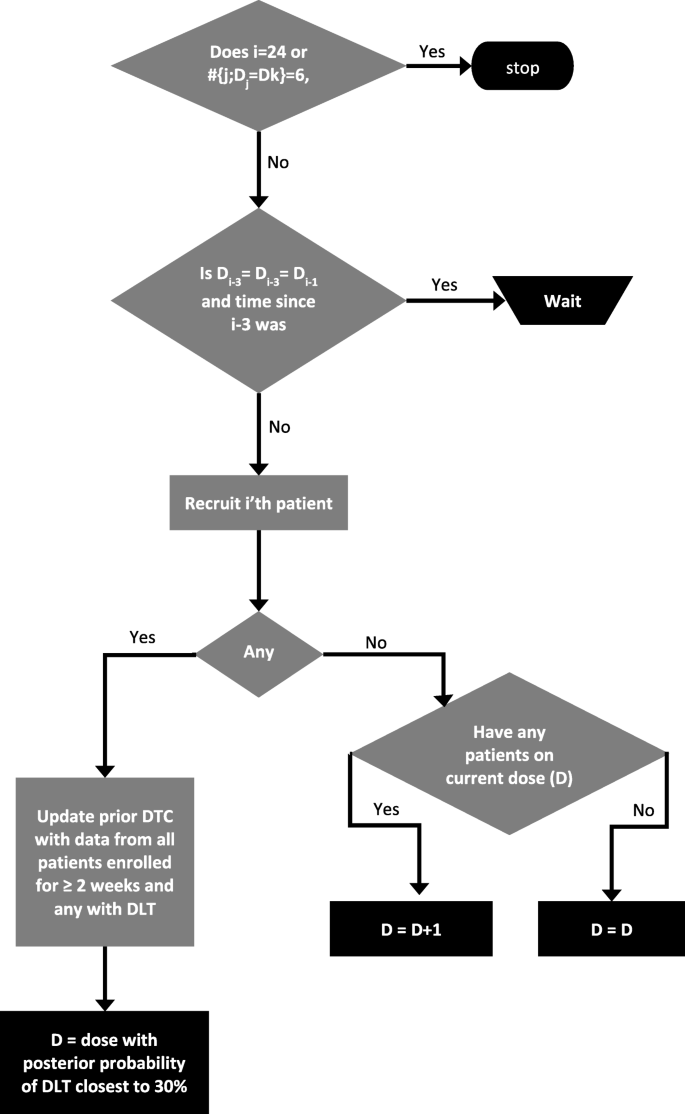
A new pragmatic design for dose escalation in phase 1 clinical trials using an adaptive continual reassessment method | BMC Cancer | Full Text

Figure 1 | Phase I dose-escalation trial of the oral AKT inhibitor uprosertib in combination with the oral MEK1/MEK2 inhibitor trametinib in patients with solid tumors | SpringerLink

Contemporary dose-escalation methods for early phase studies in the immunotherapeutics era - ScienceDirect

Contemporary dose-escalation methods for early phase studies in the immunotherapeutics era - European Journal of Cancer

Ustekinumab dose escalation: An option for nonresponsive Crohn's? | Latest news for Doctors, Nurses and Pharmacists | Pharmacy

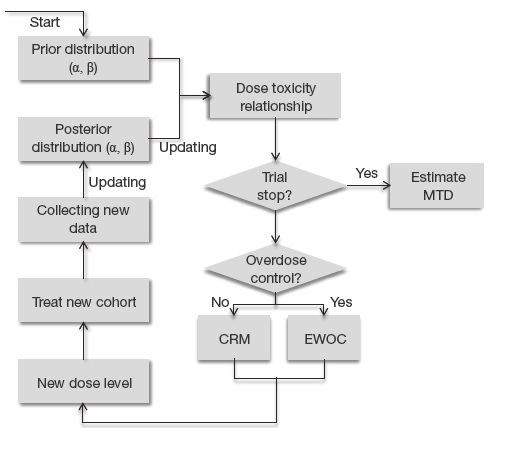

![PDF] Dose Escalation Methods in Phase I Cancer Clinical Trials | Semantic Scholar PDF] Dose Escalation Methods in Phase I Cancer Clinical Trials | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/2e565a213a4cc812f4610beb8f52eaa028d8bf12/4-Figure2-1.png)