Best Guidance On Pmcf Evaluation Report Template Doc in 2022 | Report template, Evaluation, Guidance

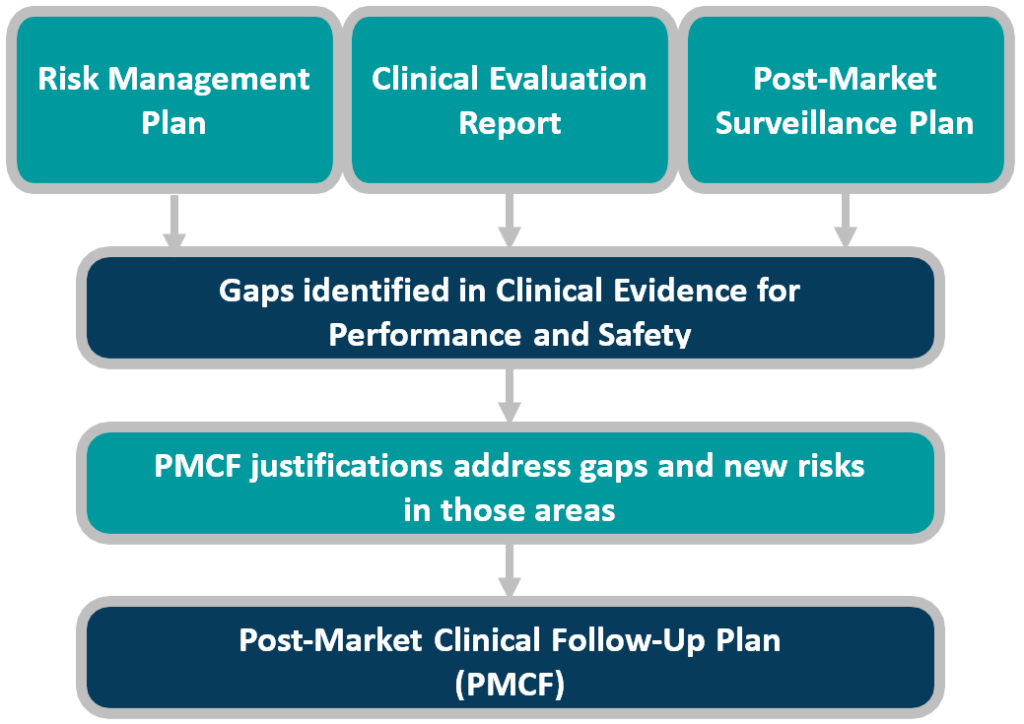
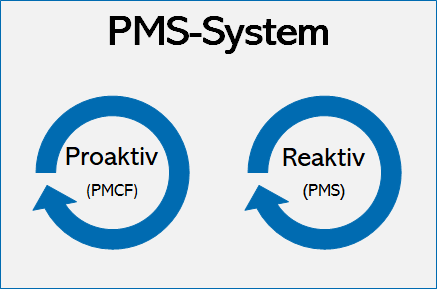
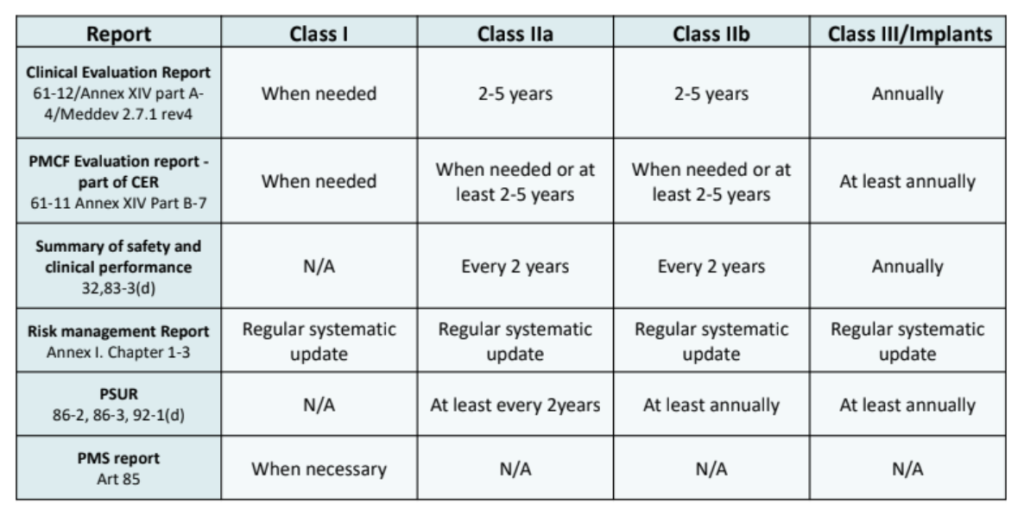
Does Your Organization's Post-Market Clinical Follow-Up (PMCF) Plan Adequately Reflect the Intensity Required in the Clinical Evaluation Report (CER) Under the Newest Medical Device Regulations? - Criterion Edge

Best Guidance On Pmcf Evaluation Report Template Doc in 2022 | Report template, Evaluation, Guidance
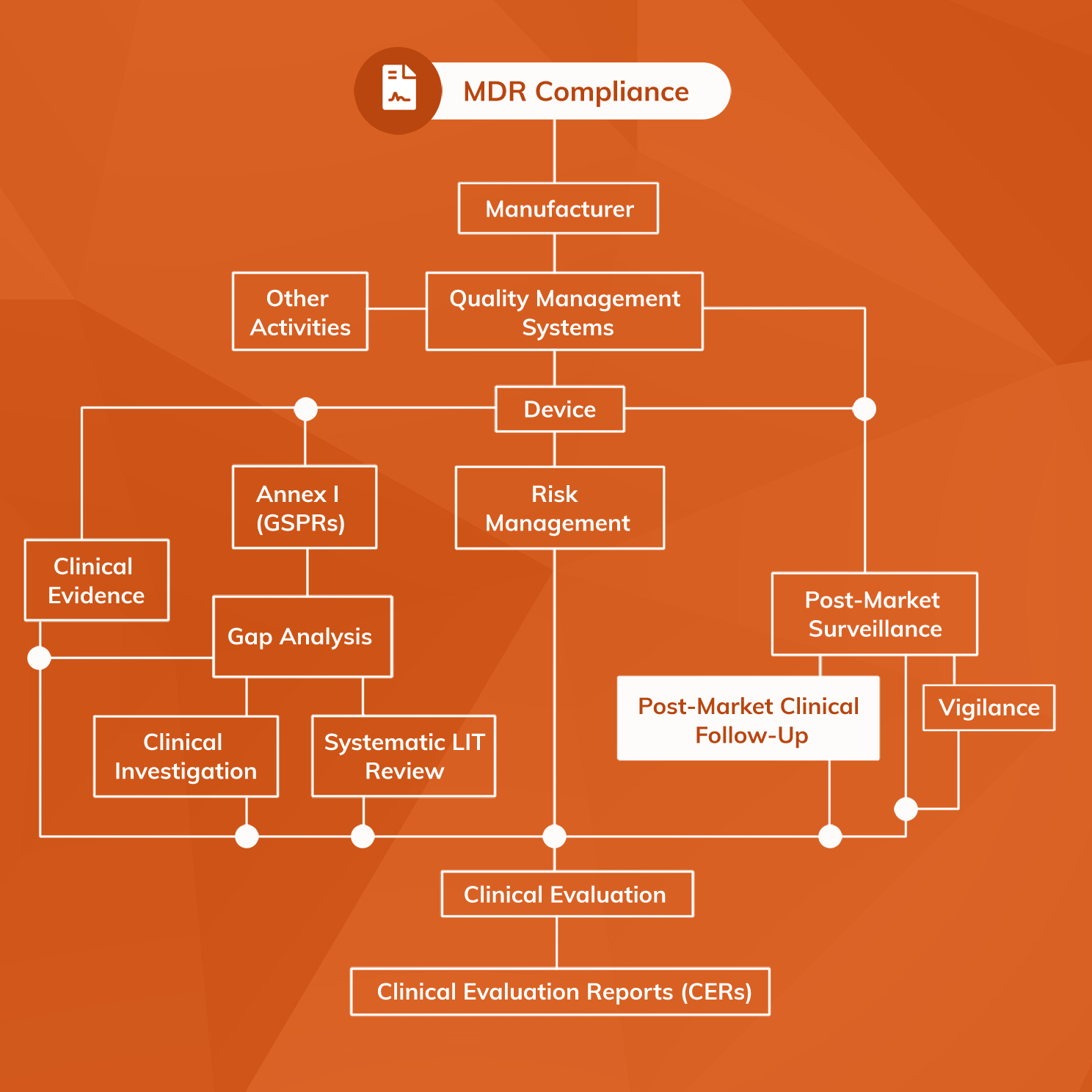
View of Getting your devices ready for MDR compliance – a clinical approach and orthopaedic device manufacturers' perspective | AboutOpen